In the BTZ combustion lab, we constantly look for new and innovative combustion techniques to change the paradigm of conventional combustion systems. In the plasmas of interest, an externally applied electric field ionizes molecules and creates high energy electrons. The high energy electrons can have very high temperatures -- e.g., several eV (1 eV is equivalent to ~10,000 K) -- and therefore can easily break stable chemical bonds to produce chemically reactive radicals. In non-equilibrium plasmas, the high energy electrons and excited species may create new pathways to produce radicals, increasing the reactivity while keeping the bulk temperature of the gas low (as low as room temperature). For example, atomic oxygen requires very high temperature to be produced in a conventional combustion system, but can be generated at room temperature with the help of non-equilibrium plasma.
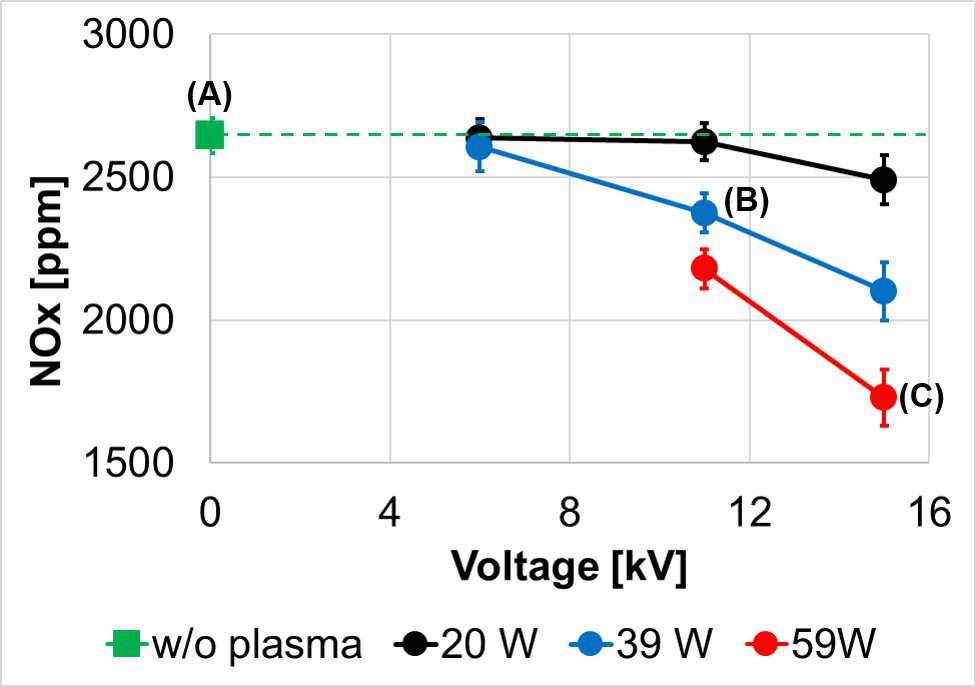
As an example, ammonia is considered as an alternative fuel. However, ammonia has very low flame speeds, which make flame stabilization difficult. Another outstanding issue of ammonia combustion is the potential of high NOx emissions. With plasma discharge, ammonia flames can be stabilized at a much leaner condition and, surprisingly, produce lower NOx emissions. (ref: Choe, J., Sun, W., Ombrello, T., & Carter, C. (2021). Plasma assisted ammonia combustion: Simultaneous NOx reduction and flame enhancement. Combustion and Flame, 228, 430-432).